15+ Calculate The Average Kinetic Energies Of Ch4 And N2
For the single-pan balance the average of the measurements is 105 g with measurements ranging from 104 g to 106 g. Download Free PDF View PDF.

Pdf Basic Principles And Calculations In Chemical Engineering Seventh Edition Anthony Valer Sanchez Academia Edu
The average kinetic energies of molecules of samples of different ideal gases at the same temperature are the same.

. Transcript 10 mol F2 440 g CO2 40 g H2 146 g SF6 34. A simplified version of Bohrs theory of the arrangement of electrons in an atom can be summarised as follows see also Figure 233. The analysis of the natural gas showed the following percentages by volume.
Download Free PDF View PDF. The molecules of a gas move in straight paths until they collide with other molecules or the walls of their containers according to the kinetic molecular theory. MEASUREMENTS DENSITY OF SOLUTION.
The operation has a design capacity of 10 loans per day and an effective capacity of 8 loans per day. Kinetic molecular theory of properties of gases 8. The electron density plots of the remaining electrons in the second energy level are very different from the spherical s orbitals.
Titration is based on a reaction between the analyte unknown sample and the regent of known concentration and reaction stoichiometry. The data and results of the. The changes in KE and PE are negligible.
Download Free PDF View PDF. Solomons graham - fundamentals of organic chemistrypdf - Academiaedu. The Brunauer-Emmett-Teller equation was used to calculate the specific surface area from adsorption data obtained at PP 0 00503.
Chemistry is often defined as the study of the _______________ The scientific method is a loosely description of how scientific knowledge is typically expanded. Intermolecular forces between the gas molecules will cause them to condense into the liquid phase if the temperature is lowered. Download Free PDF View PDF.
TITRIMETRIC MTHODS Titrimetric methods are widely used in chemistry to determine oxidants reductants acids bases metal ions etc. Download Free PDF View PDF. TITRIMETRIC MTHODS Titrimetric methods are widely used in chemistry to determine oxidants reductants acids bases metal ions etc.
137 kW 869 Reconsider Prob. For each of the following. Download Free PDF View PDF.
ABSTRACT Several model systems for the α-carbonyl proton abstraction reaction were studied at the DFT level to examine the role of hydrogen bonding interactions in the enzyme catalytic activity of acyl-CoA dehydrogenase. Our explanation of those observations is a hypothesis. Titration is based on a reaction between the analyte unknown sample and the regent of known concentration and reaction stoichiometry.
15999 g 294305 gmol mol O 1 mol C14 H18 N 2 O 5 340 102 mol C14H18N2O5 2943 g C14 H18 N 2 O 5 2943 g 459 g C14H18N2O5 mol 1g 1 mol 602 10. Processes Ideal Gas A steady flow compressor handles 1133 m 3 min of nitrogen M 28. Based on 1 mL solution.
The firm is in the 40 tax bracket and its MARR is 15. To express the concept of energy quantitatively we first have to express the kinetic and potential energies as numbers that can be added to produce a number for the total energy. Relate the rate of gas effusion with molar STEM_GC11KMT-Ij-50 gases at the molecular mass level 9.
CO2 02 and N2 08. The external surface area was calculated by the t -plot. Enter the email address you signed up with and well email you a reset link.
Electrons are in orbit around the central nucleus of the. The accompanying table gives results obtained when the object is weighed three times on each balance. In the text we define the kinetic energy of a moving object as one half of the product of the mass of the object with the square of its speed.
Study with Quizlet and memorize flashcards containing terms like 1. A loan processing operation that processes an average of 7 loans per day. 156 mol d.
Enter the email address you signed up with and well email you a reset link. Download Free PDF View PDF. Neglecting the changes in kinetic and potential energies and assuming the surroundings to be at 25C determine the reversible power input for this process.
CHAPTER 15 ACIDS AND BASES. Based on 2 mL solution. We design experiments to test the hypothesis.
14 mol C H 12011 g 10079 g 14007 g 18 mol H 2 mol N mol C mol H mol N 5 mol O b. Find the volume of air required per cum. Study with Quizlet and memorize flashcards containing terms like The diagram above is a molecular model of a gaseous diatomic element that is just above its boiling point.
Download Free PDF View PDF. K 1399 measured at intake where P1 97 KPa and T1 27 C. Discharge is at 311 KPa.
TITRIMETRIC MTHODS Titrimetric methods are widely used in chemistry to determine oxidants reductants acids bases metal ions etc. For the analytical balance the average of the measurements is 104978 g with measurements ranging from 104977 g to 104979 g. CHAPTER 15 ACIDS AND BASES.
Titration is based on a reaction between the analyte unknown sample and the regent of known concentration and reaction stoichiometry. Enter the email address you signed up with and well email you a reset link. Gases Lecture 110 Lesson 29.
Download Free PDF View PDF. The electron-density plots for these orbitals take on a shape that is best described as a dumbbell. 50 mg e.
Enter the email address you signed up with and well email you a reset link. 100 g C14H18N2O5 c. Of gas if the gas and air are at temperature of 16 C and a pressure of 1016 KPa.
Which of the following best describes how the model is limited in its. If the company plans to keep the truck for only two years what would be the equivalent present worth. Download Free PDF View PDF.
Neglect changes in kinetic and potential energies-403 KW 29. T 29615 k 337 mg CH4 148 mg Ar 210 mg of N2 PPN2 19 kPa 2. Calculate the average value of the density.
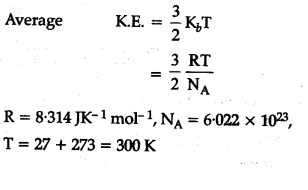
Calculate The Total And Average Kinetic Energy Of 32 G Of Methane Molecules At 27 C Cbse Class 11 Chemistry Learn Cbse Forum

Number Of Deuterium Molecules Vs Time Detected In The Gas Phase The Download Scientific Diagram

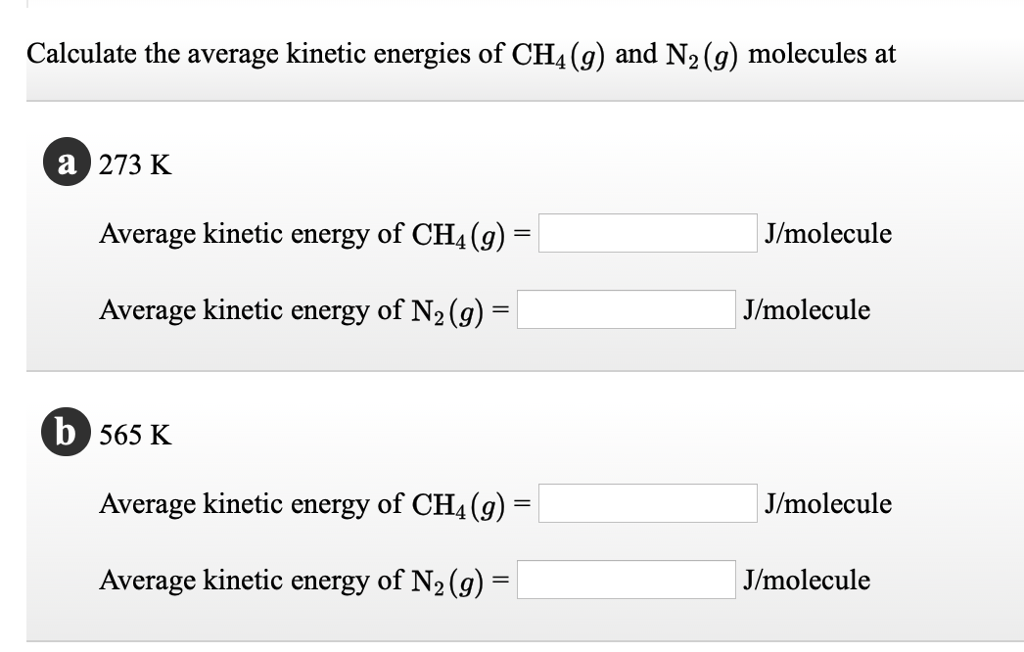
Solved Calculate The Average Kinetic Energies Of Ch4 G And N2 G Molecules At 273 K And 546 K
What Will Be The Kinetic Energy Of 4 Moles Of Nitrogen Gas At 127 C Quora
How Do You Calculate The Average Kinetic Energy Of The Ch 4 Molecules In A Sample Of Ch 4 Gas At 253 K And At 545 K Socratic
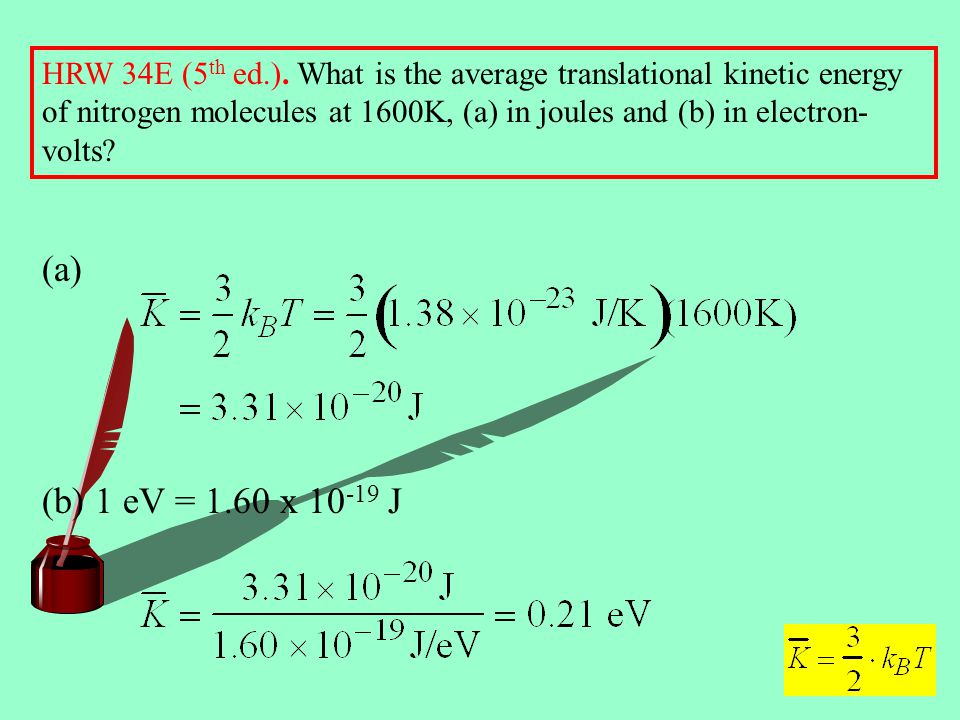
Kinetic Theory Of Gases I Ppt Video Online Download

Solved Calculate The Average Kinetic Energies Of Ch4 G And N2 G Molecules At 273 K And 546 K
How Do You Calculate The Average Kinetic Energy Of The Ch 4 Molecules In A Sample Of Ch 4 Gas At 253 K And At 545 K Socratic
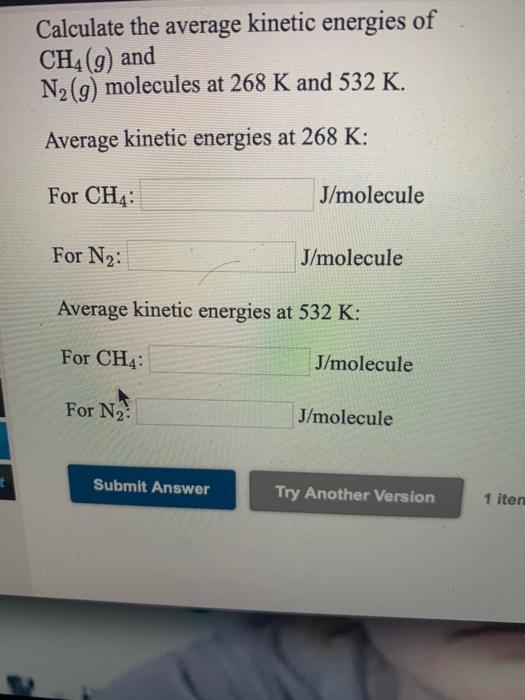
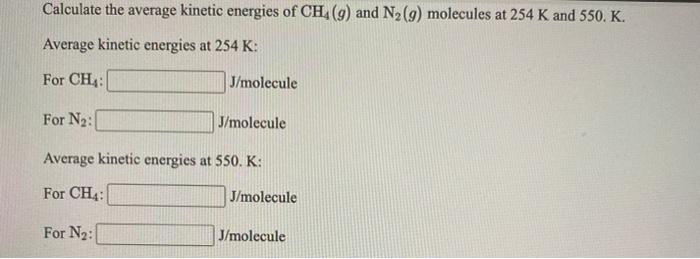
Solved Calculate The Average Kinetic Energies Of Ch4 9 And Chegg Com

Pdf An Introduction To Energy Sources Bala Kunjappa Academia Edu

Neeraj Kumar Advanced Problems In Physical Chemistry For Competitive Exami Pdf Mass Concentration Chemistry Atoms
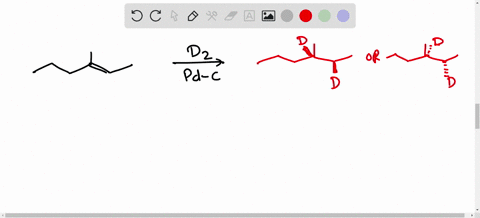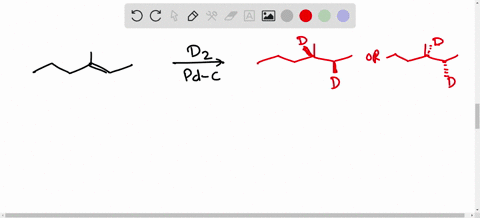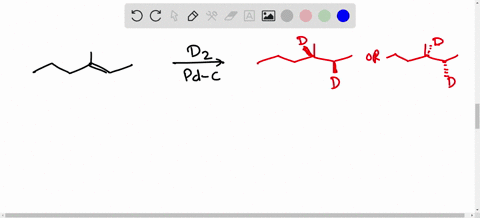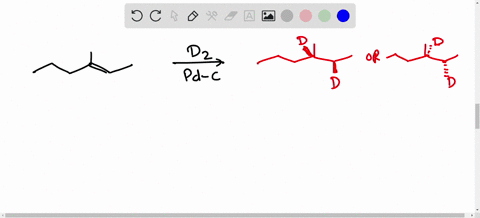
Solved Calculate The Average Kinetic Energies Of Ch4 G And N2 G Molecules At 273 K And 546 K
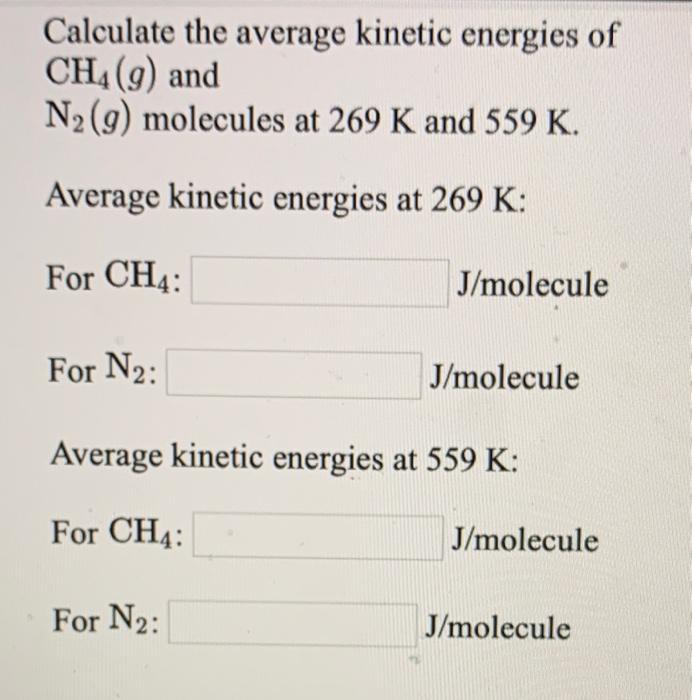
Solved Calculate The Average Kinetic Energies Of Ch4 9 And Chegg Com

A Mechanism For The Disappearance Of Propane During Methane Radiolysis
Solved Calculate The Average Kinetic Energies Of Ch4 9 And Chegg Com
Solved 77 7 Calculate The Average Kinetic Energies Of The Chegg Com
Solved Calculate The Average Kinetic Energies Of Ch4 G And Chegg Com